Written by Thomas G. Martin & Jonathan A. Kirk, Department of Cell and Molecular Physiology, Loyola University Stritch School of Medicine, Maywood, IL.
Bio:
T homas Martin is a graduate student in Dr. Jonathan Kirk’s lab at Loyola University Stritch School of Medicine in Chicago, where he uses proteomics to explore mechanisms of cellular protein quality control in cardiomyocytes. He is particularly interested in how cardiac sarcomeres are dynamically turned over in the healthy heart and how this process is impacted in heart failure to cause dysfunction.
homas Martin is a graduate student in Dr. Jonathan Kirk’s lab at Loyola University Stritch School of Medicine in Chicago, where he uses proteomics to explore mechanisms of cellular protein quality control in cardiomyocytes. He is particularly interested in how cardiac sarcomeres are dynamically turned over in the healthy heart and how this process is impacted in heart failure to cause dysfunction.
The maintenance of protein homeostasis (proteostasis) is fundamental to proteome stability and cell survival. From synthesis by the ribosome, to folding and maintenance by dozens of molecular chaperones, and finally degradation through the ubiquitin-proteasome system or autophagy, the life cycle of a protein is carefully regulated1. Dysfunctional protein quality control (PQC) is implicated in the pathophysiology of heart disease2, the number one cause of death in the United States3. However, our mechanistic understanding of cardiac PQC is relatively weak, due partially to limitations in experimental approaches, providing a significant obstacle to restoring proteostasis as a strategy for treating heart failure. Proteomics techniques offer unique opportunities to advance the understanding of cardiac PQC and help bridge the gap to treatment.
Harnessing Ubiquitinomics to Study Cardiac PQC
When proteins are damaged, often from stress-induced denaturation, they are targeted to degradation pathways to prevent cytotoxic protein aggregation. To denote which members should be removed by these pathways, the damaged proteins are tagged at lysine residues with ubiquitin. Ubiquitin-enrichment mass spectrometry is a great option for unbiased assessment of the global heart ubiquitinome and for use in more targeted studies, where the impact of disease or individual proteins on ubiquitin signaling are of interest. The technique, which was introduced a decade ago by Xu et al., relies on immunoaffinity purification of ubiquitinated substrates at the peptide level6. Specifically, when ubiquitinated proteins are digested with trypsin, the enzyme cleaves all but two amino acids of the ubiquitin moiety, leaving a diglycine lysine remnant modification. Diglycine lysine peptides can then be enriched by immunoprecipitation and identified by mass spectrometry7.
Our group recently adopted this approach to characterize how heart failure changes protein ubiquitination of the cardiac sarcomere. We found several sarcomeric proteins had increased ubiquitination in myocardial samples from human dilated cardiomyopathy, suggesting they were tagged for degradation but the clearance either stalled completely or was operating at an inadequate rate8. There are other recent examples of targeted ubiquitinomics in the skeletal muscle field, where the approach has been used to characterize substrates of the E3 ubiquitin ligase ASB2β in skeletal muscle atrophy9 and quantify changes in exercise-regulated ubiquitin signaling10. The only other examples thus far in the cardiac muscle field are studies characterizing the global heart ubiquitinome11, however these studies are few and global changes in disease have not been explored. While there has been limited use of this technique to date in the cardiovascular field, the abundance of detailed protocols and the commercially available diglycine lysine antibody make ubiquitinated-peptide enrichment a feasible approach for most laboratories with access to the necessary mass spectrometry instrumentation.
Monitoring Proteome Turnover Dynamics with Stable Isotope Labeling
Traditional molecular biology approaches such as western blot, or broader analyses like quantitative proteomics, are useful for observing changes in protein expression at a single time point. However, more sophisticated methods are necessary to explore proteome dynamics and determine whether protein expression changes arise from altered protein synthesis or protein stability/degradation. Proteomic techniques using stable isotope labeling provide a holistic assessment of protein turnover kinetics and can be much more informative to the experimenter. Using deuterium oxide (2H2O) amino acid labeling combined with high resolution mass spectrometry, Lam et al. interrogated in vivo protein turnover in a mouse model of adverse cardiac remodeling stemming from chronic β-adrenergic stimulation and found over 1,000 proteins with altered turnover kinetics12. Variations of this labeling technique and model have been used since to explore the proteome kinetics of the remodeling heart in pathological hypertrophy13,14. In a another study, Shekar et al. specifically explored mitochondrial proteome kinetics in a mouse model of heart failure secondary to trans-aortic constriction and showed that heart failure reduced mitochondrial protein content and increased the turnover rate of metabolic proteins15. Studies using stable isotope labeling in other models of heart disease remain to be performed, but it will be particularly interesting to determine how the kinetics are affected between various etiologies of heart failure, as well as in disease manifestations of genetic origin.
Beyond quantifying turnover kinetics in models of disease, the stable isotope labeling technique has been used to determine the effect of autophagy activation on cardiac proteome turnover. Dai and colleagues used deuterated leucine in combination with caloric restriction or rapamycin administration in a mouse model of aging and found that protein half-lives significantly increased with autophagy activation16. That negative age-dependent proteome remodeling can be reversed with autophagy activation is promising and one can speculate that the same may be true for heart failure, though such studies have not yet been performed.
These proteomic approaches and others are under-utilized in the study of cardiac PQC, but it is clear from recent studies how much more informative they can be when used to supplement classical approaches.
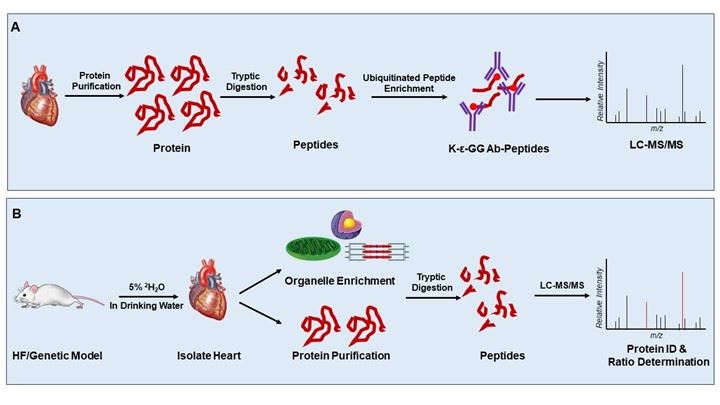
Figure 1. Basic proteomics approaches for studying cardiac PQC. A. Paradigm for purification and identification of ubiquitinated proteins from the myocardium. B. Simplified approach to studying protein turnover dynamics via in vivo stable isotope protein labeling with deuterated water.
References
1. Chen, B., Retzlaff, M., Roos, T. & Frydman, J. Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 3, (2011).
2. Martin, T. G. & Kirk, J. A. Under construction: The dynamic assembly, maintenance, and degradation of the cardiac sarcomere. J. Mol. Cell. Cardiol. 148, 89–102 (2020).
3. Dassanayaka, S. & Jones, S. P. Recent Developments in Heart Failure. Circulation Research vol. 117 (2015).
4. Willis, M. S., Townley-Tilson, W. H. D., Kang, E. Y., Homeister, J. W. & Patterson, C. Sent to destroy: The ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circulation Research vol. 106 463–478 (2010).
5. Abdellatif, M., Sedej, S., Carmona-Gutierrez, D., Madeo, F. & Kroemer, G. Autophagy in cardiovascular aging. Circ. Res. 123, 803–824 (2018).
6. Xu, G., Paige, J. S. & Jaffrey, S. R. Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat. Biotechnol. 28, (2010).
7. Udeshi, N. D., Mertins, P., Svinkina, T. & Carr, S. A. Large-scale identification of ubiquitination sites by mass spectrometry. Nat. Protoc. 8, 1950–1960 (2013).
8. Martin, T. G. et al. Cardiomyocyte Contractile Impairment in Heart Failure Results from Reduced BAG3-mediated Sarcomeric Protein Turnover. Nat. Commun. (2021).
9. Goodman, C. A., Davey, J. R., Hagg, A., Parker, B. L. & Gregorevic, P. Dynamic Changes to the Skeletal Muscle Proteome and Ubiquitinome Induced by the E3 Ligase, ASB2β. Mol. Cell. Proteomics 20, (2021).
10. Parker, B. L., Kiens, B., Wojtaszewski, J. F. P., Richter, E. A. & James, D. E. Quantification of exercise-regulated ubiquitin signaling in human skeletal muscle identifies protein modification cross talk via NEDDylation. FASEB J. 34, (2020).
11. Heunis, T., Lamoliatte, F., Marín-Rubio, J. L., Dannoura, A. & Trost, M. Technical report: Targeted proteomic analysis reveals enrichment of atypical ubiquitin chains in contractile murine tissues. J. Proteomics 229, (2020).
12. Lam, M. P. Y. et al. Protein kinetic signatures of the remodeling heart following isoproterenol stimulation. J. Clin. Invest. 124, (2014).
13. McClatchy, D. B. et al. Quantitative temporal analysis of protein dynamics in cardiac remodeling. J. Mol. Cell. Cardiol. 121, (2018).
14. Lau, E. et al. Integrated omics dissection of proteome dynamics during cardiac remodeling. Nat. Commun. 9, (2018).
15. Shekar, K. C. et al. Cardiac mitochondrial proteome dynamics with heavy water reveals stable rate of mitochondrial protein synthesis in heart failure despite decline in mitochondrial oxidative capacity. J. Mol. Cell. Cardiol. 75, (2014).
16. Dai, D. F. et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, (2014).