Michelle M. Hill, QIMR Berghofer Medical Research Institute, Brisbane, Australia and Ellen D. McPhail, Mayo Clinic, Rochester, MN, USA
Joe's Story
Following a 32-year career in plant pathology R&D for agricultural and biosecurity applications, Joe Kochman, PhD, is well-versed in DNA-based diagnostic technologies. But he never imagined that at 68-years of age, he would be the topic of a difficult diagnostic investigation which required innovations in proteomics diagnostics.
 While undergoing prostate cancer treatment in 2015, amyloid deposits were detected in Joe’s prostate gland biopsy. The chance finding led to further investigation and a diagnosis of early systemic amyloidosis with heart involvement. Joe was very fortunate that he was referred to Dr Peter Mollee, a haematologist at the forefront of systemic amyloidosis diagnosis techniques in Australia.
While undergoing prostate cancer treatment in 2015, amyloid deposits were detected in Joe’s prostate gland biopsy. The chance finding led to further investigation and a diagnosis of early systemic amyloidosis with heart involvement. Joe was very fortunate that he was referred to Dr Peter Mollee, a haematologist at the forefront of systemic amyloidosis diagnosis techniques in Australia.
“He was found to have a monoclonal lambda immunoglobulin free light chain in the blood which can be the causative protein underling AL amyloidosis.” Peter explains. “The alternative possibility was that the amyloid deposits were of transthyretin type (ATTR), and the circulating monoclonal immunoglobulin lambda free light chain was unrelated to the amyloidosis but due to condition called MGUS.”
Cardiac ATTR has a favourable survival rate compared to AL amyloidosis, with a median survival of 75 versus 11 months. As the treatments are completely different, it was important to determine the precise amyloid protein in the deposit.
The current standard diagnostic method of immunohistochemistry was conducted, but was inconclusive. Aware of this common problem and unmet diagnostic need, Peter had worked with my team to set up laser-capture microdissection-coupled tandem mass spectrometry for amyloidosis typing method at the Princess Alexandra Hospital (PAH) Amyloidosis Centre in Brisbane, Australia.
Using a section of Joe’s prostate biopsy tissue, the proteomics method identified immunoglobulin lambda light chain, as well as signature amyloid associated proteins (ApoE, SAP, ApoA4, vitronectin and clusterin). “Thus, a confident diagnosis of lambda light chain amyloidosis was made and appropriate treatment was able to be commenced.” says Peter.
Martin Middleditch, Lead Mass Spectrometry Technologist at the University of Auckland, New Zealand, is no stranger to the life-changing impact of proteomics, having typed ~140 systemic amyloidosis cases.
“We have seen many occasions where the current clinical assumption about the case based on other techniques has been overturned by our results, significantly changing the therapeutic direction.” says Martin.
Global collaboration to bring the benefits of proteomics diagnostics to all patients
Systemic amyloidosis is a rare disorder estimated to affect 5 to 13 people per million person-years. Due to the low prevalence, first-line clinicians frequently lack amyloidosis-specific experience, and getting the correct diagnosis is often a long process. However, it is crucial as an incorrect diagnosis can lead to potentially devastating outcomes.
There are 36 known types of amyloid, and establishing the correct amyloid type is critically important as prognosis and treatment vary widely depending on the amyloid type. As illustrated in Joe’s case, antibody-based methods can be inconclusive, that’s where proteomics has come to the rescue.
Since developing the laser-capture microdissection-coupled tandem mass spectrometry amyloidosis typing test for clinical use in 2008, the Mayo Clinic (Rochester, MN, USA) has typed over 20,000 amyloid specimens, and been prolific in reporting the applications and benefits of the proteomics test. As FFPE tissue blocks can be safely delivered by post, the Mayo Clinic offers proteomic service for domestic and international patients, when referred by clinicians.
Mass spectrometry-based amyloid typing is rapidly becoming the new gold standard in light of its outstanding sensitivity and specificity. Globally, the number of centres offering systemic amyloidosis typing by mass spectrometry is still limited, although interest in establishing testing centers is increasing. There are two entry barriers for new centers, namely, the cost of high-end instruments (laser capture microdissection, mass spectrometer) and the high level of laboratory and bioinformatics expertise.
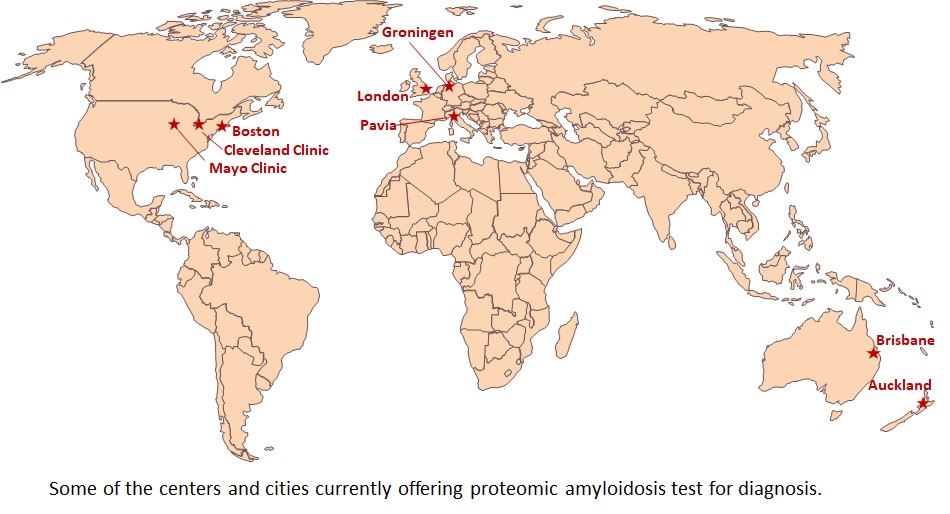
So far, all of the established centres have had successful local clinician-proteomics scientist collaboration, and mostly established their protocols independently. A harmonized international effort in education, cross-training and standardization will further broaden the availability and use of this diagnostic test to benefit more patients.
For example, abdominal fat pad aspirate is another clinically useful sample type for amyloidosis diagnosis and typing, but can present different technical challenges compared to FFPE. Cross-training with centers with greater expertise for fat aspirates, such as the University Hospital San Matteo (Pavia, Italy) or the Mayo Clinic, would facilitate the adoption and availability of global centers offering proteomics typing for amyloidosis.
Other key areas that will benefit from international collaboration and standardization include data analysis, interpretation and reporting.
Joe Kochman was lucky to have the chance biopsy finding, and referral to the only amyloidosis clinic in Australia to perform the proteomics test. He describes the events as “life changing”, and has been a patient advocate of the PAH Amyloidosis Centre since 2019.
Now an international effort in dissemination, standardization and harmonization will allow more patients globally to benefit from precision proteomics diagnosis.


.png)